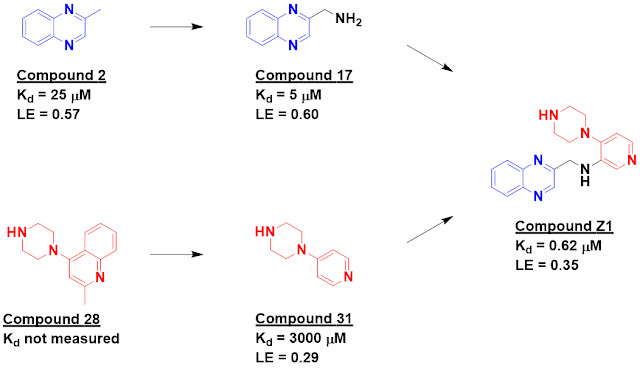
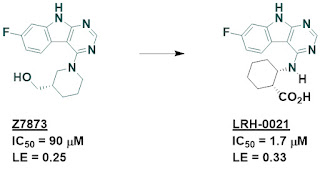
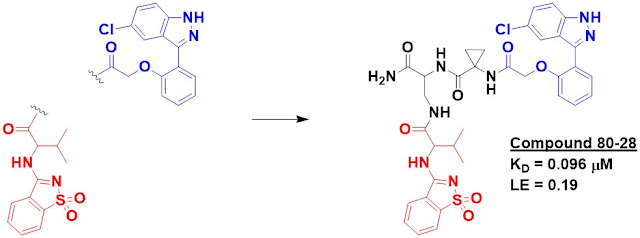
The annual
Practical Fragments look-back on the preceding year may not be the most highly anticipated year-end
tradition, but I hope you find something of interest in this twelfth edition.
I was fortunate to attend several
conferences and wrote about CHI’s
Discovery on Target in Boston and
Drug Discovery Chemistry in San Diego. As for reviews, Louise Walsh and
collaborators at Astex, Vrije Universiteit Amsterdam, Novartis, and Frontier
Medicines (me!)
published our annual analysis of fragment-to-lead success
stories in
J. Med. Chem., this one covering the year 2021. Some twenty other reviews of interest
to this readership were also published. I’ll cover them thematically below.
Methods
Crystallography is the most
popular fragment-finding technique, and in
Expert Opin. Drug Disc.
Wladek Minor and collaborators at University of Virginia and Jagiellonian
University
examine “the current role and evolution of X-ray crystallography in
drug discovery and development.” At the start of 2023 the Protein Data Bank
(
PDB) contained more than 200,000 structures, which sounds impressive until you
learn that the
AlphaFold database contains more than 200
million predicted
protein structures. But this is not experimentalist vs machine: the researchers
note how machine learning approaches can be used to more rapidly refine and
improve experimental data with resources such as
CheckMyBlob and
PDB-REDO.
For those wishing to dig deeper,
two papers in
Methods Enzymol. go into experimental detail. In the
first, Natalie Tatum and colleagues at Newcastle University describe
“crystallographic fragment screening in academic drug discovery.” May Sharpe
and collaborators at the Swiss Light Source and University of Hohenheim describe
their fast fragment-screening pipeline in a comprehensive (49 page)
guide. The
focus is on reproducibility, and there is plenty of practical advice. For
example, “the authors have even been successful in flying with crystal plates,”
though getting these through airport security may be easier in some countries than others.
Protein-detected NMR was the
first truly practical fragment-based approach, and another
paper in
Methods
Enzymol. by Brian Volkman, Brian Smith, and colleagues at Medical College
of Wisconsin describes “fragment-screening by protein-detected NMR.” This
distills eight years of effort building their internal protein-detected NMR fragment
screening platform that has been applied to 16 proteins thus far. The chapter
is particularly detailed on protein and library preparation and screening.
Compared with crystallography and
NMR, virtual screening can be dramatically faster; we’ve
highlighted multibillion-compound screens. In
WIREs Comput. Mol. Sci., Artem
Cherkasov, Francesco Gentile, and colleagues at University of British Columbia
and University of Ottawa
discuss (open access) how computational methods are
“keeping pace with the explosive growth of chemical libraries.” They cover
brute force methods, fragment-based virtual screening, and machine-learning
based methods, all while avoiding hype, and conclude that it will take time for
these methods to “have a real impact on practical drug discovery.”
Finally, Marianne Fillet and
collaborators at University of Liege and University of Namur provide a general
review in
Trends Anal. Chem. covering multiple
methods to detect
non-covalent fragments. These include established techniques such as
biochemical assays, ligand-observed NMR, crystallography, thermal shifts, and
SPR, as well as less common ones such as
WAC,
microscale thermophoresis,
ACE,
and
DEL. The paper includes several nice tables and even a decision tree to
help choose among the various approaches.
Covalent fragments
Many techniques to detect
noncovalent interactions also apply to reversible covalent inhibitors, the
subject of a
review in
Med. Chem. Res. by Faridoon and collaborators at
Genhouse Bio and Olema Oncology. The researchers focus on various warheads
including
cyanoacrylamides,
nitriles, ketones and aldehydes,
boronic acids, and
others, and provide multiple examples for each.
In contrast, an open-access
review in
Pharmaceuticals by Monique Multeder and collaborators at
Leiden University Medical Center discusses methods to detect both reversible as
well as irreversible covalent protein-drug adducts. Crystallography is the most
informative, but the researchers also delve into various mass-spectrometry
techniques including
top-down (with intact proteins) and
bottom-up (after
digestion of modified proteins). Also covered are activity-based protein profiling
(
ABPP) methods, NMR, and fluorescence-based approaches. The nearly 300
references make a useful compendium.
One of the most exciting recent
developments is “proteome-wide fragment-based ligand and target discovery,” the
subject of an open-access
review in
Isr. J. Chem. by Ines Forrest and
Christopher Parker, both at Scripps. This concise, highly readable account
covers a lot of ground, from
ABPP to fully functionalized fragments (
FFFs) to
phenotypic screening.
If you’re doing covalent FBLD
you’ll need a library of covalent fragments, and if you’re building one, I’d
recommend a
review in
Prog. Med. Chem. by David Mann and colleagues at
Imperial College London. The paper nicely summarizes design principles such as
choice of warhead and the fact that
reactivity can vary considerably even among
compounds with the same warhead. Synthetic methods and screening approaches are
also well covered, along with methods to distinguish specific binding from
nonspecific reactivity.
Most covalent fragments target
cysteine residues, but there at least nine other potentially reactive amino
acids, and these are the subject of an open-access
review by György Keserű and
colleagues at Budapest University of Technology and Economics in
Trends
Pharm. Sci. Lysine, serine, threonine, tyrosine, and histidine are the most
common targets, though some of the warheads are so reactive that specificity
will be challenging, let alone reasonable pharmacokinetic properties. This is
especially true for aspartic and glutamic acids, methionine, and tryptophan.
Finally, another
article in
Trends
Pharm. Sci. by Carlo Ballatore and colleagues at University of California
San Diego describes using covalent strategies to develop stabilizers and
inhibitors of protein-protein interactions (PPIs). Site-directed fragment
tethering with disulfide and imine chemistry is a focus, particularly in the
context of
14-3-3 proteins. Proximity-enabled covalent strategies, in which
warheads are grafted onto non-covalent molecules, are also covered. There is also
a short section on covalent PROTACs – more on that topic below.
Targets
Keeping with the theme of
protein-protein interactions, Ge-Fei Hao, Guang-Fu Yang, and collaborators at
Central China Normal University and Guizhou University
discuss fragment-based
approaches against “undruggable” PPIs in
Trends Biochem. Sci. After
describing why protein-protein interactions can be difficult, the paper presents several successful case studies, including
venetoclax,
sotorasib, and targeting
14-3-3 proteins.
Targeted protein degradation
continues to be a major
focus for drug discovery, and this is commonly achieved
by hijacking E3 ligases to cause them to ubiquitinate a target of interest. Iacovos
Michaelides and Gavin Collie (AstraZeneca) describe how FBLD has been used to
find ligands against E3s in an open-access
J. Med. Chem. paper. There
are more than 600 E3s, and because their biology relies on protein-protein
interactions they are often tough targets. Fragment hits can be weak and
difficult to advance, though the researchers do describe several success
stories including against
KEAP1 and
XIAP/cIAP.
Covalent fragments have the
potential to permanently reprogram E3 ligases, and these are covered well too.
Another difficult type of target
is RNA, the topic of two reviews. In an open-access
Curr. Opin. Struct.
Biol. paper Kevin Weeks and colleagues at University of North Carolina
Chapel Hill provide a concise and beautifully illustrated overview of the
field. They note that “RNA-targeted FBLD is in its infancy,” but given that the
first report dates to 2002 it is a long childhood, and the paper does a good
job of describing the challenges.
A more extensive
treatment of “fragment-based
approaches to identify RNA binders” is provided by Matthew Disney and
colleagues at UF Scripps in
J. Med. Chem. The paper describes many case
studies, some of which we’ve
covered, and also contains a handy table comparing
the pros and cons of a dozen different methods for finding RNA-binding
fragments.
Tuberculosis kills more than 1.5
million people each year, and fragment-based approaches have been applied
against
multiple targets within the pathogen, as
reviewed by Baptiste
Villemagne and colleagues at University Lille in
Eur. J. Med. Chem. We’ve
covered many of these studies on
Practical Fragments, but as the paper
notes none have advanced to the clinic. This is attributed in part to cell
permeability, and the researchers suggest turning to
phenotypic screens (see
below).
Other
Fragment linking can be
difficult
but highly effective, especially for difficult targets. An
overview of
published linkers is provided by Isabelle Krimm and collaborators at Université
Claude Bernard Lyon and Université Montpellier in
Expert Opin. Drug Disc.
The paper includes a table summarizing 40 fragment linking stories, noting that
most linkers are short and flexible. Another table summarizes 19 examples of
target-guided synthesis, including
dynamic combinatorial chemistry. As the paper notes, all
of these are small model studies based on known compounds. In silico
approaches, the last topic covered, will probably prove more practical.
And on the subject of practical, Dean
Brown (Jnana Therapeutics) provides an “
analysis of successful hit-to-clinical
candidate pairs” in
J. Med. Chem. This is an update to his 2018
article
and captures 156 clinical candidates reported in the journal between 2018 and
2021. Of these, 14 had fragments in their lineage. Most of these drugs appear
in our
list of fragment-derived clinical candidates (though
berotralstat
does not – I’ll need to look closer). The paper contains lots of interesting analyses.
For example, of the 138 oral drugs, 39 had a molecular weight > 500 Da, 24
had Clog > 5, and 17 had more than 10 hydrogen bond acceptors (HBA). On the
other hand, none had more than 5
HBD, emphasizing that you should be parsimonious
with hydrogen bond donors.
Finally, veteran drug hunter Nicholas Meanwell provides
“
reflections on a 40-year career in drug design and discovery” (open access) in
Med. Chem.
Rev. Those of you who saw his talk earlier this year at the
CHI DDC meeting
will know what to expect, and those of you who didn’t will be in for a treat. A
personal and entertaining romp through pharma starting in the early 1980s, the
paper is full of surprises, such as the pursuit of minor impurities in a
phenotypic screen that ultimately led to the hepatitis C drug
daclatasvir. Nicholas
notes that “you discover what you screen for, so screen design is of paramount
importance.”
The paper also reveals a passion
for medicinal chemistry: “In a search for inspiration for design concepts, I
sat down one Saturday afternoon in early October of 1987 and perused every
molecule in the United States Adopted Names (USAN) dictionary.” And, as he
notes near the end, “Decision making in drug discovery and development is a
delicate balancing act, inherently flawed based on absence of predictive accuracy,
and knowing when to conclude a discovery program with grace is also an
important trait.” That said, he provides examples of successful programs
that were almost killed multiple times – and others that were killed at Bristol
Myers Squibb but subsequently succeeded elsewhere. While this is frustrating on
one level, Nicholas takes satisfaction in the fact that “the science that we
conducted and the molecules and pharmacophores that we defined have been of
benefit to mankind.”
There are still a couple weeks
left in the year, but that’s it for Practical Fragments for 2023. Thanks
for reading, and special thanks for commenting. And if you live in one of the
70+ countries with elections in 2024, please vote.