As the year winds down SARS-CoV-2
continues its relentless drive through Greek letters and the planet. But there
is hope: vaccines seem to be holding, for those who have access, and two oral
drugs have been granted emergency use authorization by the US FDA, one of which
(
PF-07321332) is covalent and looks remarkably effective. As is our
custom,
Practical Fragments ends the year by highlighting
conferences and reviews.
This year produced more than twenty
FBLD-related reviews, and these are grouped thematically: NMR and
crystallography, computational methods, targets, library design, and covalent
fragments. The most general is the
sixth installment in a series of annual
reviews in
J. Med. Chem. covering fragment-to-lead success stories from
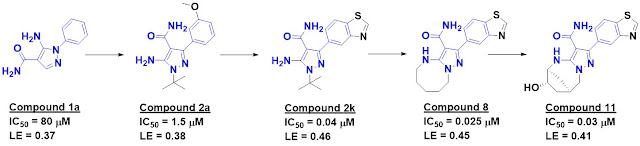
the previous year. Iwan de Esch (Vrije Universiteit Amsterdam) took the lead
(pardon the pun) on the most recent review, which details 21 examples from
2020. In addition to the centerpiece table showing fragment, lead, and key
parameters, this open-access paper also includes an analysis on the
molecular complexity
of fragment hits.
NMR and crystallography
Consistent with its central role
in FBLD, several reviews covered NMR. Ben Davis (Vernalis), one of the leading
practitioners, discusses fragment screening in
Methods Mol. Biol. The
chapter is written for a non-specialist, so you won’t see detailed pulse sequences.
Instead, Ben provides a very accessible and practical guide covering everything
from sample preparation through data analysis and validation.
A more detailed description of
solution NMR in drug discovery by Li Shi and Naixia Zhang (Shanghai Institute
of Materia and Medica) is
published (open access) in
Molecules. With 180
references, this review covers considerable ground, including various
ligand-detected and protein-detected methods for screening as well as for
hit-to-lead and mechanistic studies. The paper also includes a nice summary of
in-cell (!) NMR.
The
Pacifichem meeting had
several talks on fluorine NMR, and speaker Will Pomerantz, together with
Caroline Buchholz, has published a thorough, open-access
review in
RSC Chem.
Biol. Will has been a leading developer of protein-observed
19F
NMR, so naturally this topic is well-covered, but there is plenty on
ligand-observed
19F NMR as well as a good background section and
musings on the future of the field.
And if you’re looking for a
detailed how-to
guide for NMR-based fragment screening, Harald Schwalbe and
colleagues describe the platform they’ve built at the Center for Biomolecular
Magnetic Resonance (BMRZ) at Johann Wolfgang Goethe-University Frankfurt in
J. Vis. Exp.
This open-access paper also describes quality control experiments of the iNEXT
library, which we’ve discussed
here.
Switching gears to
crystallography,
J. Vis. Exp. carries a
paper by Frank von Delft
and collaborators describing the XChem platform at the Diamond Light Source.
This high-throughput fragment screening platform has delivered a 95% success
rate on more than 150 screens, with hit rates varying from 1-30%. In addition
to technical details, this open-access article also provides tips on
successfully getting your screening proposal through peer review.
XChem has inspired similar
efforts at other synchrotrons, including the Fast Fragment and Compound
Screening (FFCS) platform at the Swiss Light Source. This is concisely
described by May Sharpe and Justyna Wojdyla in
Nihon Kessho Gakkaishi
(open-access and published in English).
Private companies are also moving
into high-throughput crystallography. Debanu Das and collaborators describe the
platform at Accelero Biostructures, which is capable of screening ~500
fragments in two days. Screens against three nucleases are described in some
detail in an open-access
article in
Prog. Biophys. Mol. Biol.; these and
other components of the DNA damage response are the focus of XPose
Therapeutics, Accelero’s sister company.
Computational methods
In addition to the experimental
methods reviewed above, a couple papers describe computational approaches. In
Drug
Disc. Today: Tech.,
FragNet alum Moira Rachman and collaborators from UCSF, Universitat
de Barcelona, and elsewhere focus on “fragment-to-lead tailored in silico
design.” This is a nice
review of the recent literature and emphasizes the fact
that much of the heavy design lifting is still done by medicinal chemists – at least
for now.
Predicting the energies of modified
fragments has long been a challenge, and one promising approach is free energy
perturbation, in which one ligand is “perturbed” into another and the relative
energy differences calculated. Barbara Zarzycka and colleagues at Vrije
Universiteit Amsterdam provide a concise
review for aficionados in
Drug
Disc. Today: Tech.
Targets
Three reviews cover applications
of FBLD to various target classes. Kinases have been particularly successful,
with four of the six approved fragment-derived
drugs targeting these enzymes.
In
Trends Pharm. Sci., Ge-Fei Hao and collaborators, mostly at Central
China Normal University,
review the state of the art. In addition to background
and several case studies, the paper includes a nice table showing structures
and summaries of clinical-stage kinase inhibitors.
Epigenetics has been another
fruitful area, and in
J. Med. Chem. Miguel Vaidergorn, Flavio da Silva
Emery (both University of São Paulo) and Ganesan (University of East Anglia)
detail the “successful union of epigenetic and fragment based drug discovery
(EPIDD + FBDD).” This thorough summary (with 165 structures!) of the literature
is particularly detailed when it comes to bromodomains, four inhibitors of
which have entered the clinic with the help of fragments. The researchers point
out that EPIDD and FBDD both began around the same time, and in fact the oncology
drug vorinostat could be described as “a unique case of solvent-based drug
discovery.”
RNA has long been a target of
FBLD, and in
ChemMedChem Mads Clausen (Technical University of Denmark)
and collaborators
review the state of the art. The various established and
emerging methods to find fragment hits are covered in depth, and there is also
a nice discussion as to whether RNA-focused fragment libraries will be useful.
Library design and molecular
properties
The topic of three-dimensional
fragments is covered in several other reviews. In
Drug Disc. Today: Tech.,
Iwan de Esch and collaborators at Vrije Universiteit Amsterdam and University
of York
assess 25 so-called 3D libraries reported in the literature, mostly since
2015. The researchers manually drew all 897 fragments so they could calculate
various properties. While most of the molecules are
rule-of-three compliant,
just under half could be called 3D by both plane of best fit (
PBF) and
principal moment of inertia (
PMI). PBF and PMI measurements correlated with one
another, while
Fsp3 correlated with neither measurement, leading to
the conclusion that “Fsp
3 is a poor measure of 3D shape.”
Shapely or not, sp
3-rich
fragments are interesting from a diversity point of view, and in
Chem. Sci.
Max Caplin and Dan Foley (University of Canterbury)
discuss synthetic methods
for advancing these. This is an excellent open-access review of the recent literature
around C-H bond functionalization and well worth reading for the chemists in
the audience.
3D fragments are often chiral,
and the importance of chirality in drug discovery is the focus of a
paper in
ACS
Med. Chem. Lett. by Ilaria Silvestri and Paul Colbon (University of
Liverpool). The researchers note an opportunity for chemical suppliers: only
245 of 9751 heterocyclic building blocks offered by Sigma-Aldrich are chirally pure.
“Library design strategies to
accelerate fragment-based drug discovery” is the topic of a
Chem. Eur. J.
review by Nikolaj Troelsen and Mads Clausen (Technical University of Denmark).
The researchers provide a highly accessible overview of different libraries appropriate
for different fragment-finding methods, including covalent approaches.
Covalent fragments
This year saw the approval of
sotorasib,
the first covalent fragment-derived drug, so it is no surprise that several papers
focus on this topic. Sara Buhrlage, Jarrod Marto, and colleagues at Dana-Farber
Cancer Institute provide a thorough
introduction to “chemoproteomic methods for
covalent drug discovery” in
Chem. Soc. Rev. The review covers both
isolated protein screening as well as proteome-wide methods and includes multiple
case studies.
Nir London and colleagues at The
Weizmann Institute of Science focus on “covalent fragment screening” in
Ann.
Rep. Med. Chem. This is an excellent
review of the recent literature and
also includes an analysis of six commercial covalent fragment libraries.
And finally, in
RSC Chem.
Biol. (open access), Nathanael Gray and collaborators mostly at Dana-Farber
Cancer Institute
discuss strategies for “fragment-based covalent ligand
discovery”, including computer-aided approaches, as well as target classes and
new modalities such as PROTACs. They end by asking whether sotorasib was “a
lucky, one-off case” or “a preview of continued and increased impacts that
these approaches will have on drug discovery as the improved methods, larger
libraries, and increased focus start to bear fruit.”
I’m betting on the latter.
And that’s it for 2021. Thanks
for reading, special thanks for commenting, and here’s hoping we’ll be able to
meet in person in 2022.