Last week we highlighted the discovery
of a selective inhibitor for the BET family of bromodomains. The 61 human
bromodomains fall into eight subfamilies, of which the BET family has probably
been most heavily studied. In contrast, family VIII has received less attention,
in part due to the lack of selective inhibitors. This deficit is beginning to
be addressed by Shifali Shishodia, Brian Smith, and collaborators at Medical
College of Wisconsin and Purdue University in J. Med. Chem.
The researchers were particularly
interested in the aptly-named protein Polybromo-1 (PBRM1), which contains six
of the 10 family VIII bromodomains. The protein has normally been considered a
tumor suppressor, but it has also been implicated as a tumor promoter in
prostate cancer. Chemical probes would be very useful to unravel the complicated
biology. A few pan-inhibitors of family VIII have been developed, one of which
we wrote about back in 2016, but none of these are selective for the PBRM1
protein.
The researchers started with an
NMR screen of the second bromodomain of PBRM1, BD2, the structure of which had
previously been solved by NMR. A 1H-15N SOFAST-HMQC screen
of 1968 fragments (all rule of three compliant, from Maybridge and Zenobia) in
pools of 12 ultimately yielded a dozen hits, all of which are shown in the paper.
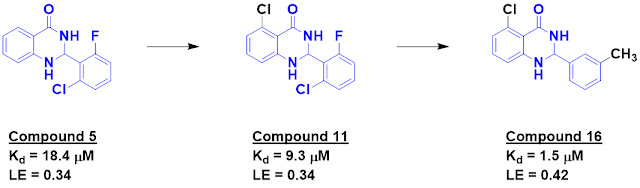
Of these, compound 5 was the most potent, with dissociation constants of 45 µM
by NMR titration and 18 µM by isothermal titration calorimetry (ITC).
One of the previous pan-family
VIII inhibitors described in the literature was structurally similar to
compound 5, and borrowing a chlorine atom from this led to compound 11, with improved
affinity. Further exploration around both phenyl rings ultimately led to compound
16, which displayed low micromolar affinity by ITC and high nanomolar activity
in an inhibition assay.
Differential scanning fluorimetry
(DSF) is commonly used to measure binding of small molecules to bromodomains,
and the researchers tested some of their best compounds in a panel of bromodomains
that included 9 of the 10 family VIII members. Encouragingly, compound 16 only
showed a strong thermal shift (ΔTm = 5.4 °C) to PBRM1-BD2 and
moderate shifts (ΔTm = 1.8 °C) to PBRM1-BD3 and PBRM1-BD5. No significant
stabilization of the 18 other bromodomains was observed.
A series of shRNA experiments by
the researchers revealed that the prostrate cancer cell line LNCaP was
dependent on PBRM1, and compound 16 was active against these cells, albeit weakly
(EC50 ~ 9 µM). In contrast, the compound did not show activity
against two other cancer cell lines that do not seem to be dependent on PBRM1.
This work is a nice example of
academic fragment-based lead discovery. Although the cell activity of compound 16 is
probably insufficient for a serviceable chemical probe, it does show that selectivity
is possible. Hopefully these researchers, or others, will continue improving
it.



No comments:
Post a Comment