Metal-binding fragments have a
long history in FBLD; the first mention on Practical Fragments was back
in 2010. The idea is to use the strong interaction between a fragment and a protein-bound
metal as an affinity anchor for further optimization. The latest example, by Jie-Young
Song, Soosung Kang and collaborators at Korea Institute of Radiological &
Medical Sciences, Ewha Womans University, and elsewhere was published in ACS
Med. Chem. Lett.
Glutaminyl cyclases such as
glutaminyl-peptide cyclotransferase (QC) and glutaminyl-peptide
cyclotransferase-like protein (isoQC) convert N-terminal glutamine or glutamate
residues on proteins to pyroglutamates. This modification tends to stabilize
proteins, and it has been implicated in several diseases. In particular, modification
of CD47 by isoQC seems to be important for the ability of cancer cells to evade the immune system.
QC and isoQC are closely
related enzymes with a zinc-containing active site. Capitalizing on this, the
researchers tested a library of 36 potential metal-binding fragments in a
functional assay against QC. Most of the compounds tested were inactive, though
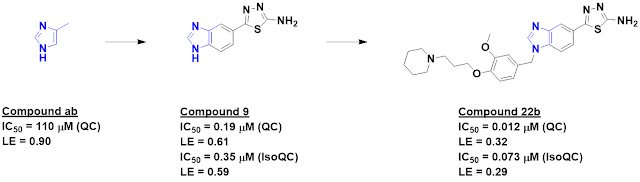
11 had IC50 values less than 0.8 mM. A few of these, including
compound ab, were used to generate a second library of just half a dozen larger
fragments, and compound 9 turned out to quite potent.
The researchers recognized that compound
9 has two potential zinc-binding moieties, and docking suggested the newly
added amino-thiadiazole was likely responsible for the increased activity.
Structure-based design ultimately led to compound 22b, with low nanomolar
activity against QC and isoQC. The molecule did not seem to be generally cytotoxic,
but it did increase phagocytosis of cancer cells in vitro, consistent with an
effect on the “don’t eat me” function of CD47.
Unfortunately, no information is
provided on the selectivity of compound 22b against other zinc-dependent
enzymes. Moreover, unlike an earlier example of starting with metallophilic
fragments, no ADME data are provided. But whether or not this particular series
advances, it is nice to see metallophilic fragments being explored.



No comments:
Post a Comment