Last week we highlighted work out
of Vernalis and Servier in which fragment-based methods were used to identify
potent and selective inhibitors of DYRK1A and 1B, potential targets for cancer
and neurodegenerative diseases. The NMR screens yielded 166 hits, only one of
which was advanced in that paper. A second J. Med. Chem. paper by Andras
Kotschy and collaborators describes the optimization of another fragment.
Compound 1 is a whoppingly potent
fragment with impressive ligand efficiency. If you’ve ever worked on kinases you
probably think you know how it binds, as the diaminopyrimidine moiety is a
common hinge-binding motif. In fact, crystallography revealed that the molecule
binds in a completely different orientation and that the methoxy group makes a
single hydrogen bond to the hinge amide NH. Cyclizing the molecule led to
compound 10, with a satisfying boost in affinity.
Unfortunately, compound 10 was also
a potent inhibitor of the kinase CKD9. To gain selectivity, the researchers
took advantage of the fact that one of the backbone carbonyl oxygens in the
hinge adopts an unusual orientation in DYRK1A, making room for the methyl group
in compound 33. Next, the researchers replaced the benzofuran core for reasons of
“synthetic tractability, metabolic stability, and freedom to operate.” This exercise
ultimately led to compound 40.
This compound was profiled
against 442 kinases and found to be quite selective, with only 8 kinases significantly
inhibited at 1 µM. One of these was the related kinase DYRK2, but further growing
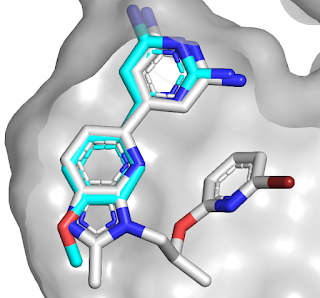
led to selective compound 58. An overlay of the initial fragment (blue) with
compound 58 (gray) reveals how the binding mode has been maintained, in
contrast to the series described last week.
Compound 40 had only modest
antiproliferative activity against human cancer cell lines that were grown in
2D culture but was more active when the cells were grown in 3D culture. The molecule
had good oral bioavailability in mice, and xenograft studies revealed that it
inhibited tumor growth, though it was also toxic at higher doses. The
researchers do not mention brain penetration, though given the number of hydrogen
bond donors I would be surprised if it crosses the blood-brain barrier.
This paper is a nice example of
how getting high affinity is often only the beginning of a long journey. In
combination with the story from last week it is also a useful reminder of how
many starting points a single fragment screen can provide: just two fragments
led to two completely independent series. Whether molecules from these series advance
to the clinic, they provide useful tools to further understand the biology of
DYRK1A.




No comments:
Post a Comment