The dual-specificity tyrosine-phosphorylation-regulated
kinases 1A and 1B (DYRK1A and DYRK1B) belong to a family of five serine/threonine
kinases implicated in several cancers as well as Down’s syndrome and other
neurodegenerative diseases. For the latter indications in particular, brain
penetration would be essential for any inhibitor, just as in the LRRK2 story
last week. In a new (open access) J. Med. Chem. paper, Rod Hubbard and
collaborators at Vernalis and Servier describe the discovery of a chemical probe.
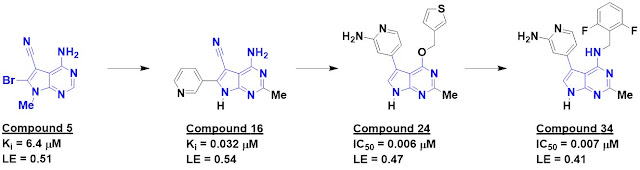
The researchers started by testing
their in-house library of 1063 fragments in pools of six, each at 500 µM, in
three ligand-detected NMR screens. This resulted in a whopping 166 hits. Crystal
structures of the eight most ligand-efficient fragments bound to DYRK1A were
obtained, including compound 5. Fragment growing led to compound 16, which
bound the kinase 200-fold more tightly.
The crystal structure of compound
16 bound to DYRK1A was compared to structures of other known ligands and suggested
the possibility for an alternative binding mode. This led to the synthesis of
compound 24, with low nanomolar affinity against both DYRK1A and DYRK1B (only
the former is shown in the figure). This compound turned out to be surprisingly
unstable in slightly acidic aqueous solution (below pH 5), but replacing the
oxygen with a nitrogen fixed this, and further tweaking ultimately led to
compound 34.
Compound 34 was profiled at 1 µM against
a panel of 442 kinases and found to be fairly selective, with only 15 kinases inhibited
by at least 50%. It is orally bioavailable in mice, brain penetrant, and
inhibited the proliferation of glioblastoma cells, although the potency was significantly
attenuated by serum. In a xenograft study the compound caused tumor growth
delays and was well-tolerated.
This is a nice example of
fragment-based lead discovery heavily dependent on structural information. Comparing
the binding mode of compound 34 (gray) with that of compound 5 (light blue)
reveals the significant shift in binding mode of the initial fragment.
The paper is also a useful
reminder of how long it can take for industry research to be published. Work
began in 2009, and Rod presented some of it at the CHI FBDD conference in 2019. But this
is not the end of the DYRK1A story: stay tuned for next week!




No comments:
Post a Comment