People living with cystic
fibrosis are susceptible to lung infections from a rogues’ gallery of bacterial
species, one of which is Mycobacterium abscessus. It is often antibiotic
resistant, and even when it responds, a course of antibiotics can take two
years to resolve the infection. In a recent ACS Infect. Dis. paper Tom
Blundell, Anthony Coyne, and collaborators at University of Cambridge and
elsewhere describe progress against this organism.
The researchers chose to target a
protein called SAICAR synthetase, or PurC, which is essential for purine biosynthesis
and thus bacterial growth, as shown by genetic knockout studies. The enzyme is
significantly different from the human ortholog, but similar to the Mycobacterium
tuberculosis ortholog, giving the potential for a twofer.
Fragment screening was conducted
both in-house using thermal shift assays as well as at XChem using crystallography;
we discussed the differing outputs of these screens in this 2019 post. Compound
1, from the in-house screen, was found crystallographically to bind in the
ATP-binding site, and ITC studies revealed it to have high micromolar affinity
for the protein. Meanwhile, compound 2 was identified from the crystallographic
screen, and while the affinity wasn’t measured, the pyridyl ring is located a
short distance from where compound 1 binds.
Initial SAR around fragment 1 revealed
that growing toward the binding site of compound 2 would be possible, as
illustrated by compound 9. Appending a pyridyl ring onto this molecule led to compound
16, with low micromolar affinity. The pyridyl moiety stacks onto an arginine
side chain, and improving this interaction by replacing the pyridyl with a phenyl
appended with electron-withdrawing fluorine atoms led to compound 27, with
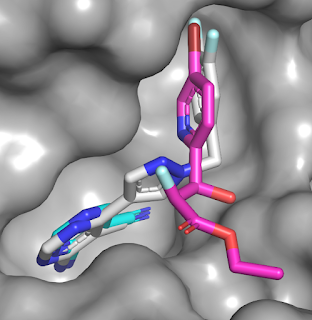
submicromolar activity. Overlaying the crystal structures of compounds 1 (cyan),
2 (magenta), and 27 (gray) reveals that the merged molecule does indeed bind in
a similar manner to the component fragments.
Unfortunately, despite good
biochemical activity against PurC, none of the compounds were particularly effective
at inhibiting growth of either M. abscessus or M. tuberculosis. Such
disconnects between biochemical and cell potency are unfortunately all too common,
particularly for antimicrobial targets, as we wrote about here. The researchers
suggest possible reasons including efflux and physicochemical properties. The
paper ends by noting that work is continuing, and we look forward to hearing
more.




1 comment:
The electron-withdrawing fluorine atoms may not be very important for affinity, as the phenyl group itself also led to submicromolar binding. On the other hand, replacing 3-fluoro with 3-trifluoromethyl appeared to be detrimental for affinity as reflected by TSA.
Post a Comment