Last week we highlighted Steve
Fesik’s presentation at the Discovery on Target meeting in which he discussed the
discovery of a covalent inhibitor of the oncology target KRASG12C. The paper describing this
work, by Joachim Bröker, Alex Waterson, and collaborators at Boehringer
Ingelheim and Vanderbilt University, has just appeared (open access) in J. Med.
Chem.
A decade ago we described how the
Fesik lab reported finding millimolar fragments that bind to the so-called switch
I/II pocket on KRAS. In collaboration with researchers at Boehringer Ingelheim,
these were optimized to sub-micromolar ligands that block nucleotide exchange (see here).
However, these molecules hit all RAS isoforms and show only modest cell
activity. In contrast, the approved drug sotorasib binds in a different pocket,
called switch II, and forms a covalent bond with an oncogenic cysteine
mutation, G12C.
To find molecules that would bind
in the switch II pocket, the researchers first needed to block the switch I/II
pocket, which seems to be a hot spot for fragment binding. They did so by
introducing a cysteine mutation near the pocket and linking this via a
disulfide to a small fragment. All this was done in the context of KRASG12V,
a mutant that is more common in cancer than KRASG12C. The modified protein
was then screened using two-dimensional protein-observed (HSQC) NMR against 13,000
fragments. This process identified 20 fragments that bind outside of the switch I/II
pocket, including compound 1, which bound to the modified protein with
mid-micromolar affinity.
A combination of SAR-by-catalog
and synthesis suggested the importance of both the amino group and the nitrile,
and these observations were confirmed by a crystal structure of compound 1
bound to the protein deep in the switch II pocket, as predicted from the NMR
data. The crystal structure also revealed a vector to grow the molecule,
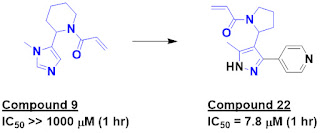
leading to compound 12. This molecule had sufficiently high affinity to bind to
KRASG12V without the introduction of the switch I/II blocking fragment.
Further growing to compound 19 and addition of a phenyl group (compound 20b)
led to low micromolar binders. Installation of an acrylamide warhead and further
decoration led to BI-0474, which rapidly reacted with the mutant
cysteine in KRASG12C. Interestingly, the initial fragment is carried
through unchanged.
In addition to potent biochemical
activity, BI-0474 showed low nanomolar cell activity. The “bioavailability was
not yet optimized,” but intraperitoneal administration led to anti-turmor
activity in mouse xenograft models. The paper also notes that “a more advanced
orally available analogue from this series has recently entered phase I
clinical trials.” As we noted earlier this year, this is BI 1823911.
It is worth contrasting this work
with the discovery of sotorasib, which we discussed in 2020. Sotorasib traces
its origins to covalent fragment screens, and an electrophile was maintained
throughout the optimization process. In contrast, the new paper starts with a non-covalent
fragment that was optimized before an electrophilic warhead was
introduced. This is probably more typical of how covalent drugs are discovered, as exemplified last year for the BTK inhibitor TAK-020. However, it is not necessarily
easy; Steve mentioned in his presentation earlier this month that achieving the
optimal configuration of the warhead took some effort.
KRAS has become a poster child
for the power of fragment-based approaches to deliver drugs against
previously intractable targets. The fact that the new molecules have good non-covalent
affinity broadens the range of ligandable oncogenic mutants beyond KRASG12C. Indeed, the researchers end by noting that their approach
has led to “molecules that are highly attractive for further use in the
discovery of inhibitors against other KRAS mutants.”
Let’s hope they – and others –
succeed.