1.2 million deaths. If you did
not read the title of this post carefully you may assume this statistic refers
to COVID-19. In fact, it is the number of people who died of tuberculosis in
2019. Worse, drug resistant forms of Mycobacterium tuberculosis, the organism
that causes TB, are spreading far faster than new treatments are being
developed. Initial efforts at addressing this problem are reported (open access)
in Comp. Struct. Biotech. J. by Sangeeta Tiwari (University of Texas El
Paso), Vitor Mendes (University of Cambridge) and a multinational team of
collaborators.
M. tuberculosis is capable
of making all 20 amino acids. The bug can also scavenge arginine from its host,
but only inefficiently: knocking out the biosynthetic pathway abolishes virulence.
Thus, targeting this pathway might lead to new drugs.
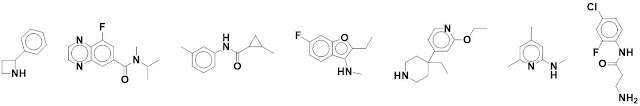
In total eight enzymes are needed
to synthesize L-arginine from L-glutamate, and the researchers targeted four of
them. The proteins were screened against a library of 960 fragments (each at 5
mM) using differential scanning fluorimetry (DSF). Depending on the specific
target some of the hits were validated by SPR or ligand-based NMR before being
taken into crystallography, which yielded structures of all the enzymes.
In total 13 fragments were found to bind to ArgB, 4 bound to ArgC, 2 bound to
ArgD, and 8 bound to ArgF. All the coordinates have been deposited in the
protein data bank, though they don’t seem to have been released as of June 28.
The paper details the binding
interactions for all the hits. Most of them are quite weak, though two hits
against ArgB have low micromolar dissociation constants as assessed by ITC. Tantalizingly,
these inhibit the growth of M. tuberculosis, and one of them seems to be on-target (adding arginine to the media rescues the inhibition). All the ArgB
fragments bind not at the active site but rather at an interface between
protein subunits. Unfortunately this site is quite hydrophobic, as are the
fragments, suggesting an uphill battle in optimization.
A good antibiotic should not hit
human proteins, and neither ArgB nor ArgC have human orthologs. ArgF does, but
the region where the fragments bind is quite different. ArgD, with only two
crystallographically-confirmed hits and 36% identity to the human enzyme, is
probably the least attractive.
A year before the COVID-19 Moonshot launched we highlighted the Open Source Antibiotics initiative. I don’t
think that team was involved with this research, but they would seem to be a
natural fit. If you have spare bandwidth and are looking to do some fragment to
lead optimization, this paper provides more than two dozen starting points.