One of the more exciting phenomena in
fragment-based approaches is synergy (or superadditivity), in which the binding
energy of linked fragments is greater than the sum of the binding energies of
the individual fragments. Extreme cases are relatively rare, and the underlying
thermodynamics can be counterintuitive, so it is always fun to see new
examples. Cosimo Altomare and collaborators at the University
of Bari and Consiglio Nazionale delle Ricerche
(Italy
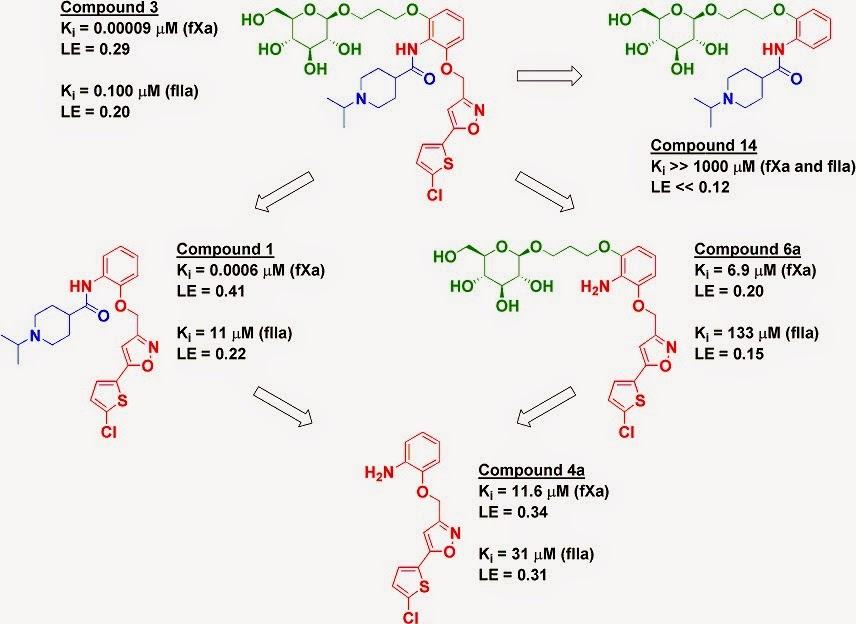
The proteases factor Xa (fXa) and thrombin
(fIIa) are two heavily-studied anticoagulant targets. The paper characterizes a
previously described molecule (compound 3) that is selective for fXa but still
potent against fIIa, leading to good anticoagulant activity in human plasma as
well as profibrinolytic activity. The researchers took a fragment deconstruction approach to better understand the binding to both targets.
As seen previously for fXa, the chlorothiophene
moiety (red) is essential for binding, and removing it (compound 14)
obliterates any detectable activity on both enzymes. However, while removing
the glucose moiety (green) to give compound 1 reduced affinity for fXa by less
than ten-fold, it reduced affinity for fIIa by more than two orders of
magnitude. In contrast, removing the piperidine moiety (blue) to give compound
6a reduced affinity to both enzymes by several orders of magnitude.
However, these results are
context-dependent. Removing both the piperidine moiety and the glucose moiety gives compound 4a, which has similar
activity against fIIa as compounds 1 and 6a, where only a single moiety has
been removed. In fact, compound 4a (without the glucose) is actually slightly more potent than compound 6a (with the
glucose) against fIIa. But, as mentioned above, adding the glucose to compound 1 gives an impressive 110-fold boost in affinity for
fIIa. In comparison, a famous early example of cooperativity in an NMR by SAR
study gave only a 14-fold boost.
The researchers solved the crystal
structure of compound 3 bound to fIIa, which reveals several hydrogen bond
interactions between the glucose moiety and amino acid residues that have been
previously implicated in allosteric activation of the protein. Perhaps compound
3 is exploiting this allosteric mechanism to bind more tightly.
This is a careful, thorough study and
serves as a useful reminder that cooperativity can be huge, but it is still
difficult to explain, much less predict.



No comments:
Post a Comment