Our last post discussed the growing plague of molecular obesity, and how numerous metrics have been designed to control it. In a paper published online in J. Comput. Aided Mol. Des. Paul Mortenson and Chris Murray of Astex describe a new one: LLEAT.
Although ligand efficiency (LE) is probably the most widely used and intuitive metric, it does not take into account lipophilicity. Other indices do, notably ligand lipophilicity efficiency (LLE) and ligand-efficiency-dependent lipophilicity (LELP), but these both have drawbacks for evaluating fragments. LLE (defined as pIC50 – log P) is not normalized for size; for a fragment to have an (attractive) LLE ≥ 5 it would need an exceptionally low log P or an exceptionally high affinity. LELP, defined as log P / ligand efficiency, is also potentially misleading since a compound could have an acceptable LELP value even with a low ligand efficiency if the log P is also very low.
To address these problems, Mortenson and Murray have tried to strip out the non-specific binding a lipophilic molecule experiences when going from water to a binding site in a protein. They define this modified free energy of binding as:
ΔG* = ΔG - ΔGlipo
≈ RT ln (IC50) + RT ln (P)
≈ ln (10) * RT (log P - pIC50)
In order to put values coming out of this metric on the same scale as those from ligand efficiency, they add a constant, such that:
LLEAT = 0.11 – ΔG* / (number of heavy atoms)
Thus, just as in ligand efficiency, the goal is for molecules to have LLEAT ≥ 0.3 kcal/mol per heavy atom.
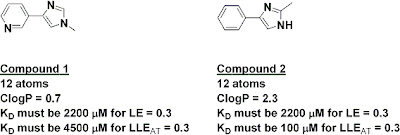
The index has some interesting implications. For example, the two fragments below have the same number of heavy atoms, and thus if they had the same activity they would have the same ligand efficiency; on this measure alone, neither would be preferred as a starting point for further work. However, because of their very different lipophilicities, fragment 2 would need to be 45 times more potent than fragment 1 in order to have the same LLEAT of at least 0.3.
A similar analysis can be done during optimization. For example, adding either a phenyl or a piperazinyl substituent should produce a 20-fold boost in potency in order to maintain ligand efficiency at 0.3, since both have 6 atoms. However, in order to maintain LLEAT at 0.3, the phenyl would need to produce a 460-fold boost in potency while the piperazinyl would need to improve potency only 3-fold. This is consistent with what other folks have reported qualitatively, but it’s nice to have a simple quantitative measure.
Although some people may groan at yet another index, and no metric is perfect, I like the fact that this one is intuitive and has the same range of “acceptable” values as ligand efficiency. What do you think – is it useful?



6 comments:
I'd actually discussed something similar to this a couple of years ago in a blog post:
http://fbdd-lit.blogspot.com/2009/06/scaling-potency-by-lipophilicity-and.html
In general I am not a fan of converting potency and/or affinity data to free energies (I prefer to work with pIC50 or pKd) because the units of energy are frequently omitted. Also quoting delta-G obscures the nature of the data (are IC50s being mixed with Kds?).
I'd be interested to know what the measured octanol/water logP values were for compounds 1 and 2. Interactions between the rings of compound 1 may weaken both acceptor nitrogens and the ClogP algorithm might not pick this up.
It's also worth remembering that octanol/water is not the perfect partitioning system that a lot of people make it out to be. I would guess that Compound 2 would actually be less lipophilic in an alkane/water partitioning system.
So here's my question to the authors of this paper. Does a hydrophobic pocket look more like (wet) octanol or alkane?
I think Dan's right that there may be too many ligand efficiency type metrics and I think not many of them (including this one) are especially clever or are suitable for rigorous theroretical examination. For this reason we'd been pretty reticent about publishing on this (although Paul had presented it as a poster at the UK RSC Fragments conference in March 2009). What swung it for us was that we have been using it quite a lot in fragment to lead work and our chemists find it a useful way of flagging up that fragments are too lipophilic for their size and potency. Peter's points about the deficiencies of cLogP and octanol water coefficients are well made but frankly, even within our own company, I'm not sure we could reasonably use any other measure of lipophilicity and expect it to widely adopted. What we're aiming for here is a simple rule of thumb that might have some utility in guiding decision making.
I think the approach proposed by Chris and Paul is a sensible way to bring together size and lipophilicity into a single efficiency metric (especially when you look at alternatives such as LELP). I should point out that we do not yet have general access to alkane/water partition coefficients so for now we're stuck with octanol/water. I'll also admit that I've used both pIC50/N(HevAtom) and pIC50-ClogP (even before it got christened LLE) in analysis of HTS output. It's also possible that octanol/water logP 'works' better in congeneric series in which polar groups are often relatively conserved than when performing analysis on more diverse structures.
Nevetheless, if I were presented with equal Kd values for compounds 1 and 2 and advised on basis of lipophilicity that 1 was 'better' then I would disregard the advice.
What do other people think? Any thoughts on LELP, for example? What about compounds that are predominantly charged under assay conditions?
I’ve been asking around at various conferences, and many people report using ligand efficiency, even medicinal chemists with no exposure to fragments. In contrast, I don’t recall meeting anyone who says they use LELP or other metrics. I’m struck by Chris’s comment that the chemists at Astex have been finding LLEAT useful. It makes sense that because LLEAT scales with LE, it is easier to adopt than a whole new metric would be. I’ll be interested to know whether this catches on outside Astex.
I’m not sure I agree about disregarding lipophilicity data in choosing between compounds 1 and 2. Obviously it’s not the only factor to consider, but given some of the recent publications (and a lot of anecdotal data) it seems like focusing on less lipophilic fragments is a good idea.
I do agree with Pete that the theory behind metrics can get a bit squishy; charged compounds won’t be treated properly for calculating ClogP, and IC50 values will probably be used interchangeably with Ki values. Still, as an intuitive and easy to calculate approximation of non-lipophilic binding energy per heavy atom, it seems like LLEAT could be pretty helpful.
All other things being equal, I would favor the less lipophilic of two equipotent fragments of the same size. My point is that I do not regard ClogP (octanol/water) as a relevant measure of lipohilicity for compounds 1 and 2. In some situations (e.g. naphthalene and quinoline) ClogP will be more relevant). In some ways the choice of compounds 1 and 2 is unfortunate because they represent precisely the sort of situation in which one needs to switch on the physical organic chemistry and switch off the metrics.
As I conceded earlier, we'll be stuck with octanol/water for a while yet. That doesn't mean that we should ignore its deficiences as a partitioning system and be prepared to over-ride it when making decisions. As an aside, the link between lipophilicity and bad outcomes is not as strong as some creative ways of analysing data might suggest.
"IC50 values will probably be used interchangeably with Ki values":
This will be partially true only for special cases (that is, to follow Michaelis-Menten type of experiment in the case of Ki). Even though, magnitude values will be off-scaling. To circumvent this, some folks have suggested corrections to be made. Please, see Comparability of Mixed IC50 Data – A Statistical Analysis
Tuomo Kalliokoski*, Christian Kramer*, Anna Vulpetti, Peter Gedeck
PLoS One April 2013 | Volume 8 | Issue 4 | e61007
Post a Comment