As many of us know all too well,
traditional methods to treat cancer often result in severe and even intolerable
side effects. An emerging, gentler approach is based on synthetic lethality:
targeting a protein that is essential only in certain cancer cells but not in normal
cells. One prominent target is MAT2a, one of two human methionine
adenosyltransferases. We’ve written previously about AG-270, a fragment-derived
MAT2a inhibitor that entered the clinic. AstraZeneca has also pursued this
target, as we discussed here. In a new J. Med. Chem. paper, Stephen
Atkinson, Sharan Bagal, and their AstraZeneca colleagues describe a new
chemical probe.
A differential scanning
fluorimetry (DSF) screen of about 55,000 compounds at 100 µM, nearly a third of
which were fragments, resulted in a healthy 1.5% hit rate. Further DSF as well
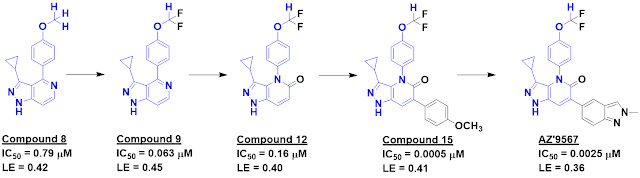
as biochemical testing ultimately delivered compound 8, which is quite potent
for a fragment. A crystal structure of the compound bound to MAT2a demonstrated
that it bound in the same allosteric site targeted by other compounds. The
methoxy group was pointed towards a couple backbone carbonyl oxygen atoms, and adding
a couple fluorine atoms created a weak hydrogen bond donor with a satisfying 50-fold
boost in potency.
Adding a hydrogen bond acceptor (compound
12) slightly reduced potency but also decreased lipophilicity. Further
inspection suggested opportunities for fragment growing, and free energy
perturbation (FEP) calculations suggested that adding the methoxyphenyl group
of compound 15 would be fruitful. This turned out to be the case, and further optimization
led to AZ’9567. The paper provides plenty of meaty medicinal chemistry, with significant
efforts focused on reducing lipophilicity and clearance. FEP was used
extensively during the design process, and a retrospective analysis found a
good correlation between predicted and measured affinity.
AZ’9567 was studied in considerable
detail. It has excellent oral bioavailability and good pharmacokinetics in both
mice and rats. The compound does not significantly inhibit cytochrome P450 enzymes
or hERG and is reasonably clean against a panel of 86 off-targets. The main liability
is poor solubility, a problem also faced by AG-270. Nonetheless, the
AstraZeneca researchers were able to develop a liquid formulation.
The paper compares AZ’9567 with
AG-270, showing that both compounds are potent in biochemical assays as well as
against cell lines in which MAT2a is essential. A mouse xenograft model with AZ’9567
showed considerable and sustained tumor growth reduction.
Unfortunately, AG-270 is no longer
in clinical development, and there is no mention of a MAT2a inhibitor in the AstraZeneca
pipeline. Nonetheless, having a second well-characterized chemical probe will
be useful for further characterizing the biology of MAT2a and assessing whether
it will be a productive drug target.
No comments:
Post a Comment