Among fragment-finding methods,
ligand-based NMR ranks near the top in terms of popularity. Of its many variations,
fluorine (19F) NMR appears to be gaining in popularity. Fluorine NMR
has several advantages, including high sensitivity and the fact that many fragments
can be screened simultaneously because of the wide chemical shift range for
fluorine. Although more commercial fluorine-enriched libraries are available
now than when we first wrote about the approach a decade ago, the diversity of
these libraries is still somewhat limited. This problem has been tackled by
Mads Clausen at the Technical University of Denmark and an international team
of collaborators in a new Angew. Chem. Int. Ed. paper.
The researchers wanted to create a
fluorinated fragment library that would be not just diverse but also contain a
high fraction of sp3-hybridized carbons (high Fsp3). Some
of the early claims around “three dimensional” fragments have been questioned, and
there seems to be little if any correlation between the shapeliness of fragments and
that of derived leads, but if you’re going to make new fragments in academia it
makes sense to explore interesting molecular architectures.
Starting from just six simple building
blocks, each containing a trifluoromethyl group, the researchers generated nine
different cores which were further derivatized at multiple positions to yield
115 diverse fragments. Consistent with diversity-oriented synthesis, no more
than five synthetic steps were used for any molecule. All molecules were made
as racemates in order to further increase the diversity of the library.
The resulting “3F Library” is
mostly rule-of-three compliant, though given that the trifluoromethyl moiety
alone adds 69 Da the fragments do tend to be larger, with an average molecular
weight of 284 Da. They are, however, less lipophilic than two commercial fluorinated
fragment libraries. And with an average Fsp3 = 0.7 and 3.3 chiral centers
they are also quite shapely as assessed by principal moment of inertia.
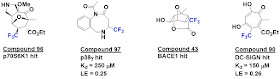
Building a library is nice, but
will it provide hits? To find out, the researchers screened the 102 fragments
that passed quality control against four targets. They used a transverse (T2)
relaxation assay (specifically, CPMG) in which fragments bound to a protein tumble
more slowly, causing a reduction in 19F signal intensity. Hit rates
ranged from 3% to 11%, and about two thirds of these confirmed in STD or WaterLOGSY
assays. As seen by the examples shown here, the fragments are quite diverse.
Whether these hits will lead to
more potent molecules remains to be seen. Laudably the paper ends with the statement:
“we hope that the 3F library will find use for other researchers and we
encourage anyone interested in screening the fragments to contact us.” If you are
looking for interesting new fragments that are tailored for follow-up chemistry,
I encourage you to take the team up on their offer.
Dear Dan
ReplyDeleteThank you for highlighting our 3F Library and for the nice comments. We agree completely that it will be interesting to see if these starting points will lead to better ligands in the years to come. For now we were encouraged to see that different scaffolds came up as hits for the four proteins we screened.
Hi Dan, although I criticized the study in which Fsp3 was introduced, I agree that getting more saturated carbon atoms into fragments is a good design theme. I would argue that the rationale for doing so is based more on molecular diversity than the idea that Fsp3-rich fragments are inherently better compounds than Fsp3-poor fragments. In the correlation inflation article that you’ve referred to, we actually suggested biasing substituents towards axial positions, with minimal steric footprint, as a molecular design theme. My view of the featured library is that many of the fragments appear to be of relatively high molecular complexity and this is likely to compromise their ability to cover chemical space in an optimal manner.
ReplyDelete