The Mycobacterium genus of
bacteria contains more than 190 species, including the pathogens that cause
leprosy and tuberculosis. Mycobacterium abscessus (Mab) is not as
famous, though it is a growing concern for people living with cystic fibrosis.
The enzyme tRNA (m1G37) methyltransferase (TrmD) is essential for Mycobacterium
growth. In a recent open-access paper in J. Med. Chem., Chris Abell, Tom
Blundell, Anthony Coyne, and collaborators at University of Cambridge, Royal
Papworth Hospital, and the US NIH describe potent inhibitors of this target.
The researchers screened 960
fragments using differential scanning fluorimetry (DSF). The 53 hits were soaked into
crystals of TrmD, and 27 yielded electron density – all in the cofactor (S-adenosyl
methionine, or SAM) binding site. One weak but particularly ligand-efficient
fragment was advanced through fragment growing to low micromolar affinity, but further
improvements proved challenging.
A powerful feature of FBLD is
that screens often yield multiple starting points, and this proved useful here.
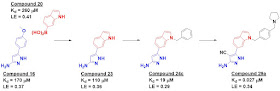
Specifically, compound 16 bound in the pocket where the adenine of SAM normally
sits, while compound 20 bound nearby in the ribose pocket. Merging these led to
compound 23, and a crystal structure suggested
that the indole nitrogen would be a good vector for fragment growing, resulting
in compound 24c. Addition of a positively charged moiety to engage a couple glutamic
acid residues led to compound 29a, a mid-nanomolar compound as assessed by isothermal
titration calorimetry (ITC).
Several of the molecules were
characterized by native electrospray ionization mass spectrometry (ESI-MS) – an
interesting but somewhat controversial technique in which protein-ligand
complexes are gently ionized and detected in the gas phase. For large molecules
such as proteins, even the gentlest ionization will generate highly charged
species; in this case the dimeric TrmD protein had between 13 and 17 positive
charges. Each of these charge states represents a different species, or perhaps
several since the positive charges could be on different regions of the
protein.
Compound 24c has modest affinity,
but encouragingly, native ESI-MS at 100 µM ligand revealed two bound ligands,
as expected for a protein dimer. However, for the much more potent compound 29a,
native ESI-MS at 100 µM ligand revealed two bound ligands for a high charge
state but largely unbound protein for a lower charge state. The researchers speculate
this could be due to dissociation of the positively charged ligand in the gas
phase.
Overall this paper is a nice exercise
in fragment merging and growing, guided by crystallographic data. Some of the
molecules elaborated from 24c (including 29a) inhibited growth of Mycobacteria
species, though further improvements in affinity will be needed, and no DMPK
properties are provided. Still, it's good to see solid work being done on new antibacterial agents.
The disconnect between observed affinity by native ESI-MS and that observed by ITC does make one cautious about the utility of the former technique. We’d welcome comments on your experiences with it.
The disconnect between observed affinity by native ESI-MS and that observed by ITC does make one cautious about the utility of the former technique. We’d welcome comments on your experiences with it.
No comments:
Post a Comment