People often
wonder how selective fragments need to be. According to molecular complexity theory, the answer is “not very”. After all, it would be hard to get a decent
hit rate with a library of just a few thousand fragments if they were too
selective. In the case of kinases, experimental studies support this theory.
Indeed, a single fragment has given rise to several drugs – one of which is
approved. In a new paper in J. Med. Chem.,
William Shipe and colleagues at Merck demonstrate the utility of a non-selective fragment for
another class of enzymes, phosphodiesterases (PDEs).
The human genome
contains more than 50 different PDEs, which cleave phosphodiester bonds. PDE10A
hydrolyzes cyclic guanosine monophosphate (cGMP) and cyclic adenosine
monophosphate (cAMP) and is a potential target for schizophrenia. It has been
pursued extensively, both with fragments (see for example here and here) as
well as more traditional approaches.
The researchers
started with a biochemical assay that screened each fragment at 200 µM; 60 of
the 1600 tested gave > 80% inhibition. Nine of these were soaked into PDE10A
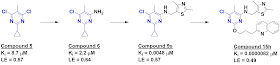
crystals, producing seven structures, including compound 5, with impressive
potency and ligand efficiency. Initial SAR by catalog led to the even more
potent compound 6, which revealed that an amino group was tolerated and pointed
nicely towards another pocket, offering a way for further elaboration.
Fragment growing
from the amino group was accomplished through several rounds of parallel
synthesis, with crystallography used to understand and optimize the binding
interactions. Compound 9s showed particularly impressive low nanomolar potency,
as well as at least 80-fold selectivity against nine other PDEs. In contrast,
the initial fragment 5 was at most only 11-fold selective against any of the
other PDEs.
Previous work
with PDE10A had revealed another “selectivity pocket” nearby, and the researchers further grew their
molecule towards this, leading ultimately to compound 15h, with low picomolar
affinity and at least >5900-fold selectivity against nine other PDEs. The
compound also showed functional activity in a rat model, though it suffered
from suboptimal pharmacokinetic properties.
This is a
beautiful illustration of the power of combining fragment screening,
structure-based drug design, and parallel synthesis. The researchers were able
to gain more than a million-fold improvement in potency and take a marginally selective
fragment to a highly selective lead. Of course, there is still plenty of work
to do, and it will be fun to watch this story unfold.
Beautiful fragment growth and SAR!!! very excited when I read this paper. I have presented it in the JC this morning. Enjoyed!!!
ReplyDelete