Practical
Fragments rarely has guest bloggers, but we do make
exceptions in special cases. What follows is a (lightly edited) analysis from
Darren Begley that appeared on the Emerald blog last year, but since the
company's transformation to Beryllium it is
impossible to find. This post emphasizes how important it is to carefully analyze commercial compounds. (–DAE)
In a LinkedIn Discussion post, Ben
Davis posed the following question:
Do
any of the commercially available fragment libraries come with reference 1D NMR
spectra acquired in aqueous solution?
Most commercial vendors of fragments do not
offer nuclear magnetic resonance (NMR) reference spectra with their compounds
useful to fragment screeners; if anything, the experiment is conducted in 100%
organic solvent, at room temperature, at relatively low magnetic field strength
(DAE: though see here for an exception). The NMR spectra of fragments and other
small molecules are greatly affected by solvents, and can vary from sample to
sample. Different buffers, solvents, temperatures and magnetic field strengths
can generate large spectral differences for the exact same compound. As a
result, NMR reference spectra acquired for fragments in organic solvent cannot
be used to design fragment mixtures, a common approach in NMR screening.
Furthermore, solubility in organic solvent is no measure of solubility in the
mostly aqueous buffer conditions typically used in NMR-based fragment
screening.
At Emerald [now Beryllium], we routinely
acquire NMR reference spectra for all our commercially-sourced fragment
screening compounds as part of our quality control (QC) procedures. This is
necessary to ensure the identity, the purity and the solubility of each
fragment we use for screening campaigns. These data are further used to design
cocktails of 9-10 fragments with minimal peak overlap for efficient STD-NMR
screening in-house.
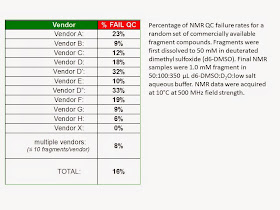
Recently, we selected a random set of commercial
fragment compounds, and closely examined those that failed our QC analysis. The
most common reason for QC failure was insolubility (47%), followed by
degradation or impurities (39%), and then spectral mismatch (17%) (Since
compounds can acquire multiple QC designations, total incidences > 100%.)
Less than 4% of all compounds assayed failed because they lacked requirements
for NMR screening (that is, sufficiently distinct from solvent peaks or lack of
non-exchangeable protons). Failure rates were as high as 33% per individual
vendor, with an overall average of 16% (see Figure).
These results highlight the importance of
implementing tight quality control measures for preliminary vetting of
commercially-sourced materials, as well as maintaining and curating a fragment
screening library. They also suggest that 10-15% of compounds will fail quality
control, regardless of vendor. Do these numbers make sense to you? How do they
measure up with your fragment library?
Let us know what you think. (–DB)
Our QC protocol was somewhat different but the outcome was very similar. Initially we dissolved our fragments to 200 mM in D6-DMSO and from that prepared an NMR sample at a concentration of 1 mM in phosphate buffered D2O (50 mM phosphate, pD 7.4) and 1% D6-DMSO.
ReplyDeleteSamples failed QC on the basis of lack of solubility in aqueous buffer or DMSO, <90% purity or having a spectrum inconsistent with the expected structure.
Just under 30% of compounds failed QC, with solubility as the major reason for failure.
Since I first posted this, a few companies have started to do their own NMR-friendly solubility studies. I believe there are at least two vendors who are going to offer 1D 1H spectra acquired in standard ligand-observe NMR screening conditions. Not only a great test for solubility and purity, but useful for assembling into pools, if that is something of interest.
ReplyDelete