The three members of the Pim family of serine/threonine kinases help cancer cells proliferate and survive, and are thus interesting as potential anti-cancer targets. Structurally the kinases are also intriguing because they have a proline residue in the “hinge” region of the purine binding site, differentiating them from the other 500+ kinases in the human genome. Two recent papers describe different fragment-screening approaches against Pim-1: one case rediscovers fragments of a previously reported compound and the other identifies a new series of potent inhibitors unlike other reported kinase inhibitors.
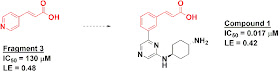
In the first paper, published in Acta Cryst. Section D, researchers from Bayer Schering Pharma AG performed a crystallographic screen of just 36 fragments against Pim-1. Despite the small library size, a dozen of the fragments produced interpretable electron density, although only 4 of these showed activity better than 10 mM. Fragment 3, with an IC50 of 130 micromolar in an enzymatic assay, was the most potent. Interestingly, a close analog of this cinnamic acid fragment was also part of a Pim-2 inhibitor previously identified through HTS by a group from Boehringer Ingelheim. The Bayer folks synthesized this molecule (compound 1), confirmed that it is also potent against Pim-1, and found that, crystallographically, it overlays beautifully with the fragment.
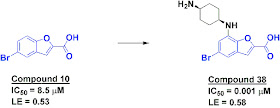
The second paper, published in Bioorg. Med. Chem. Lett., describes how researchers at Genzyme used a fragment-based approach to develop potent Pim-1 inhibitors. The researchers used SPR to screen a library of about 1800 fragments at 75 micromolar concentration and then used a biochemical assay to characterize the active molecules. Benzofuran-2-carboxylic acids such as compound 10 (below) were particularly interesting: not only did they have high ligand efficiencies, they don’t look anything like typical kinase inhibitors. X-ray crystallography revealed that the bromine atom is binding in a hydrophobic pocket, while the acid is making hydrogen bond contacts with the catalytic lysine and other residues. A related fragment with comparable potency had a methoxy substituent in the 7-position, and by transforming this into a positively charged moiety the researchers were able to improve the potency to low nanomolar with a dramatic increase in ligand efficiency.
What’s also interesting is that if you overlay the two fragments from the two papers, they superimpose almost exactly on top of one another, with the carboxylic acid moieties making the same interactions – clearly a hot spot.
On a side note, as most readers are probably aware, sanofi-aventis has recently acquired Genzyme. Practical Fragments wishes all the folks in Massachusetts well during the integration.

Thanks for the well wishes Dan. So far so good with the integration, but it's early days. Keeping fingers (and toes) crossed...
ReplyDeleteThis comment has been removed by a blog administrator.
ReplyDelete