Among fragment-derived drugs that
have entered the clinic, BACE1 inhibitors are well-represented. Sadly, multiple
drugs targeting this protein have failed to show efficacy against Alzheimer’s
disease. That said, every drug that has been thrown at Alzheimer’s has failed
to slow the disease, so perhaps we need to think more boldly. An example was published
recently in J. Med. Chem. by Andrew
Petros, Eric Mohler, and colleagues at AbbVie.
The researchers were interested
in apolipoprotein E4 (apoE4), one of three isoforms found in humans. Folks who
have two apoE4 alleles are at increased risk for Alzheimer’s, suggesting that
the protein might make a good drug target. Unfortunately, although it is known
to be a lipid carrier, its precise function is unclear. What is known is that apoE4 is less stable to
thermal denaturation than apoE2 or apoE3, so the team set out to find molecules
that would stabilize the protein. This being AbbVie, they used two-dimensional
NMR to find fragments.
The methyl groups of all the isoleucine,
leucine, methionine, and valine residues in apoE4 were 13C labeled,
and the researchers looked for changes in the 13C-HSQC spectra upon
addition of fragments; just over 4000 were screened in pools of 12, each at 1
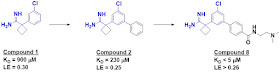
mM. Of the dozen or so hits, compound 1 was among the best.
NMR titration studies revealed an
affinity just under 1 mM, while SPR suggested slightly stronger binding. As
hoped, compound 1 raised the melting temperature of apoE4. Adding the fragment
also altered the kinetics of liposome breakdown, causing the protein to behave
more like apoE2 and apoE3. Although this assay isn’t necessarily
physiologically relevant, the reasoning is that causing apoE4 to behave more
like the other isoforms may be useful.
A crystal structure of compound 1
bound to apoE4 revealed the fragment to be binding in a small pocket, and
growing led to compound 2, with a slightly improved affinity. Introduction of
polar substituents to interact with a nearby aspartic acid side chain led to compound
8, with low micromolar affinity (assessed by NMR). This molecule also
stabilized apoE4 with respect to thermal denaturation.
As noted above, it is not
entirely clear why apoE4 is associated with Alzheimer’s, but researchers had
previously found that overexpression in a neuronal cell line caused release of the
inflammatory cytokines IL-6 and IL-8. When human induced pluripotent stem cell
(iPSC)-derived astrocytes carrying two copies of apoE4 were treated with compound
8, release of IL-6 and IL-8 cytokines was reduced to levels similar to those
from iPSC-derived astrocytes carrying two copies of apoE3. The compound also showed
no toxicity, even at relatively high concentrations (100 µM).
There is still a tremendous amount
to do: affinity needs to be improved considerably, and permeability is also mentioned
as an issue. Moreover, the highly polar nature of compound 8 will likely make
transport across the blood-brain barrier challenging. Optimizing activity
against a target whose function is poorly understood will present a host of
problems. But if it were easy, Alzheimer’s disease would not be the scourge
that it is. Practical Fragments
salutes thinking outside the box, and wishes those involved the best of luck.
I recall seeing an apparently successful paper doing what sounds very much like this 10 or more years ago. What has happened to this approach in the interval?
ReplyDeleteGood question - anyone else know?
ReplyDeleteBut relevant to the discussion, Feng Zhang (MIT) has just reported using CRISPR to change APOE4 to APOE2.
Found it:
ReplyDeletehttps://www.ncbi.nlm.nih.gov/pubmed/23013167