Last year we described the
discovery of asciminib, an allosteric inhibitor of the kinase BCR-Abl that
binds in the enzyme’s myristoyl-binding pocket. As we also highlighted nearly a
decade ago, molecules that bind in this pocket can either inhibit or activate
the enzyme. Although inhibitors have the most obvious therapeutic potential as
anti-cancer agents, activators of the ubiquitously expressed c-Abl protein
could potentially treat chemotherapy-induced neutropenia. In a recent J. Med. Chem. paper, Sophie Bertrand and
coworkers at GlaxoSmithKline describe their efforts in this area.
The researchers started with a high-throughput
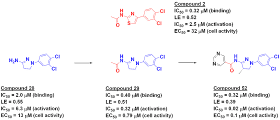
screen of 1.3 million compounds. Among the hits was fragment-sized compound 2,
which showed good binding and activation in biochemical assays but only modest
activity in cells. Building off the left side of the molecule improved biochemical
potency, but cell activity still lagged. SAR studies on the dichlorophenyl
moiety suggested that this hydrophobic group was probably optimal, and a
crystal structure of an analog bound to the enzyme confirmed this. Replacing
the central thiazole with other aromatic rings also did little to improve cell
activity.
The researchers acknowledge “that
the chemistry strategy was largely pursuing compounds with rather poor physical
properties,” notably low solubility, high lipophilicity, and high aromatic
character. As co-author Robert Young has noted previously, physical properties
matter. Happily, a fragment screen identified compound 28.
Adding the acetyl group from the
HTS hit generated compound 29, with improved activity compared to the fragment.
Moreover, this molecule had better solubility and permeability compared to the
more lipohilic, thiazole-containing compound 2. Compound 29 also showed
significantly improved activation of c-Abl in a cellular assay. Crystallography
revealed that it bound in a similar fashion as compound 2, but with a twisted,
more “three-dimensional” shape.
Further optimization, in part
informed by previous work done on the thiazole series, ultimately led to
compound 52, the most active compound synthesized. Another molecule in the
pyrazoline series showed good pharmacokinetic properties in mice. Unfortunately,
in vivo efficacy studies had to be halted early due to unexpected (and not
clearly understood) toxicity.
This paper nicely illustrates
several points. First, the power of fragment-assisted drug discovery, in which
information from both HTS and FBLD is combined for lead optimization. Second,
the inherently fuzzy line between FBLD and other discovery approaches: had
compound 28 been tested in the HTS collection, it likely would have been a hit.
Third, the importance of physicochemical properties. And finally, the
inadequacy of potency and physicochemical properties alone to produce a
developable compound. You can optimize your molecule to the best of your
ability but still be sideswiped by nasty surprises such as toxicity. It is
helpful to be clever in drug discovery, but you need to be lucky too.
No comments:
Post a Comment