Human lipoprotein-associated
phospholipase A2 (Lp-PLA2) is an enzyme involved in lipid metabolism
that is implicated in multiple diseases, from atherosclerosis to Alzheimer’s.
Because the natural substrates are lipophilic phospholipids, it is no surprise
that reported inhibitors are also large and hydrophobic. A case in point is
darapladib: with a molecular weight of 667 Da and a clogP of 8.3 this is a
poster child for molecular obesity – and it also failed in phase 3 clinical
trials. A new paper in J. Med. Chem.
by Alison Woolford (Astex), Joseph Pero (GlaxoSmithKline) and colleagues
describes an effort to discover less lipophilic inhibitors.
The researchers performed a
screen of 1360 fragments using thermal shift and ligand-detected NMR and a smaller
screen of 150 fragments using crystallography. This yielded 34 fragments that
were ultimately characterized crystallographically; screening commercial and
in-house collections for related fragments yielded another 16. Interestingly,
rather than clustering at a single hot spot, these fragments bound to different
regions of the extended active site, with some – such as fragment 6 – a full 13
Å from the catalytic center. This is reminiscent of a fragment campaign against
soluble epoxide hydrolase, another enzyme with a long, hydrophobic active site.
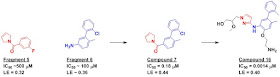
In addition to binding in an
interesting site, fragment 6 also has good affinity and ligand efficiency.
Moreover, its binding site overlaps partly with that of fragment 5. Thus, the
researchers merged the two fragments together, resulting in compound 7, with
submicromolar activity.
Further structure-guided
optimization, which included growing into a polar region of the protein,
ultimately led to compound 16, with low nanomolar potency.
Compound 16 has a molecular
weight of 411 Da and a clogP of 3.4 and is correspondingly reasonably soluble
(> 0.3 mM). Whereas darapladib showed a dramatic 700-fold potency decrease
upon addition of human plasma – presumably due to nonspecific binding to other
proteins – the decrease in potency for compound 16 is only 13-fold. Indeed, though darapladib is a picomolar binder, compound 16 is slightly more potent in plasma.